MYL and partner Fujifilm Kyowa Kirin Biologics Co Ltd. ABBV announces that the US.
Pfizer Wins Fda Approval For Biosimilar Of Abbvie S Humira Biospace
NORTH CHICAGO Ill Feb.

When was humira fda approved. HUMIRA Receives FDA Approval For Treatment of Crohns Disease ABBOTT PARK IL USA -- Abbott announced today it has received US. Below is a brief description of the studies and their results. Food and Drug Administration FDA approved HUMIRA.
Announced that the FDA has approved a biosimilar of blockbuster drug Humira adalimumab-fkjp under the brand name Hulio. Yes First approved December 31 2002 Brand name. Food and Drug Administration FDA approved HUMIRA.
ABBV today announced that the US. The approval comes at a time when HUMIRA the companys blockbuster rheumatoid arthritis treatment is getting set to. ABBV today announced that the US.
Feb 24 2021 1914 ET. December 31 2002 Persons with disabilities having problems accessing the PDF files below may call 301 796-3634 for assistance. AbbVies HUMIRA wins FDA approval for ulcerative colitis AbbVie NYSE.
Humira is the first biologic treatment to receive FDA approval for this condition since 1999 and the first to be administered by injection in these patients once every two weeks. NORTH CHICAGO Ill Feb. Yes First approved July 6 2020.
HUMIRA safely and effectively. NORTH CHICAGO Ill Feb. Humira when used as directed has been shown in clinical trials to be effective at reducing important symptoms of each of the conditions for which it is approved.
See full prescribing information for HUMIRA. 24 2021 PRNewswire -- AbbVie NYSE. HUMIRA adalimumab Receives FDA Approval to Treat Pediatric Patients Living with Moderately to Severely Active Ulcerative Colitis Published Feb 24 2021 714PM EST.
Food and Drug Administration FDA approval to market HUMIRAR. FDA has approved HUMIRA adalimumab for the treatment of moderately to. 24 2021 PRNewswire -- AbbVie NYSE.
Humira was approved by the FDA in 2002 and its core patents expired in 2016 according to the Biosimilars Council a division of the Association for Accessible Medicines which represents generic. HUMIRA adalimumab Injection Solution for Subcutaneous use Initial US. Humira was approved in December 2002 and is manufactured by AbbVie Inc.
58 Zeilen Action Type. In July the European Commission approved Humira for the treatment of active moderate to severe hidradenitis suppurativa acne inversa in adults with an inadequate response to conventional systemic hidradenitis suppurativa treatment in the European Union. Of North Chicago Illinois.
13 Zeilen Humira FDA Approval History.
Https Www Accessdata Fda Gov Drugsatfda Docs Bla 2014 125057orig1s280 Pdf
 Fda Approves Sandoz S Biosimilar Of Humira Hyrimoz
Fda Approves Sandoz S Biosimilar Of Humira Hyrimoz
 Humira Adalimumab Fda Package Insert Drug Facts Iodine Com
Humira Adalimumab Fda Package Insert Drug Facts Iodine Com
 Biospace Feature A Look At Miracle Drug Humira S Journey To Proven Efficacy Biospace
Biospace Feature A Look At Miracle Drug Humira S Journey To Proven Efficacy Biospace
Fda Approves Humira Biosimilar That Won T Be Available Until 2023 Biospace
 Ibd Medication Avsola Biosimilar To Remicade Approved By Fda
Ibd Medication Avsola Biosimilar To Remicade Approved By Fda
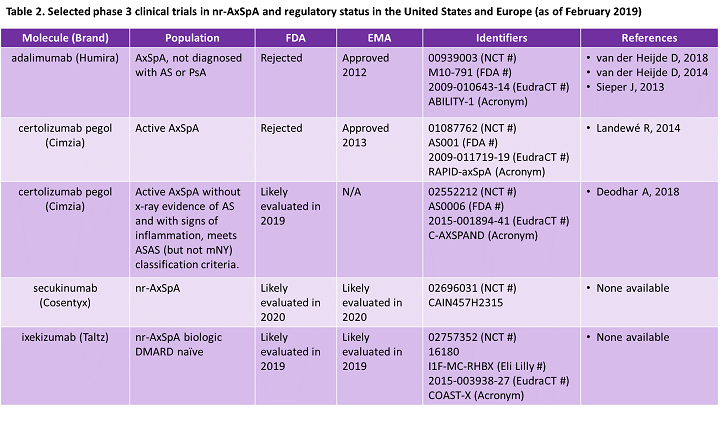 Fda Approval Of The First Nr Axspa Biologics Projecting Regulatory Market Dynamics
Fda Approval Of The First Nr Axspa Biologics Projecting Regulatory Market Dynamics
 Fda Approves Lower Cost Alternative To Biotech Drug Humira Chicago Tribune
Fda Approves Lower Cost Alternative To Biotech Drug Humira Chicago Tribune
 Eu Official Leans On Ema To Release Abbvie Data On Humira Fiercepharma
Eu Official Leans On Ema To Release Abbvie Data On Humira Fiercepharma
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2008 125057s0110lbl Pdf
 Fda Approves Hulio Sixth Humira Biosimilar
Fda Approves Hulio Sixth Humira Biosimilar
Fda Approves The First Humira Biosimilar Pharmatimes
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.