Innovative Drugs A recent widely circulated estimate put the average cost of developing an innovative new drug at more than 800. As such it teaches the vital managerial.
 Changing R D Models In Research Based Pharmaceutical Companies Journal Of Translational Medicine Full Text
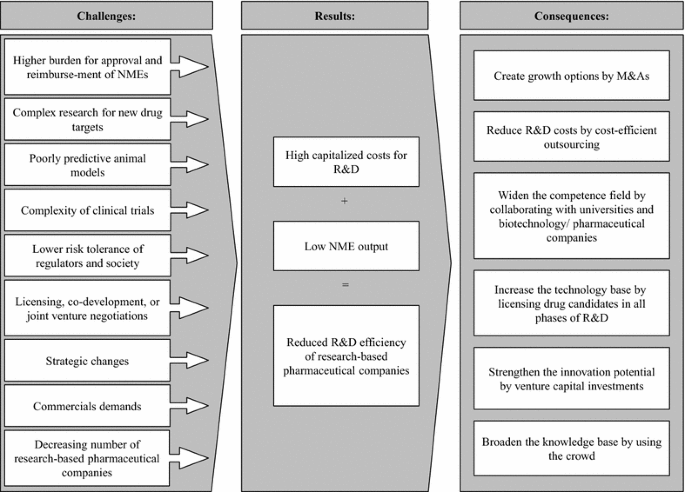
Changing R D Models In Research Based Pharmaceutical Companies Journal Of Translational Medicine Full Text
In 2019 the pharmaceutical industry spent 83 billion dollars on RD.
:max_bytes(150000):strip_icc()/research_pharma-5bfc322b46e0fb0051bf11a0.jpg)
What is research and development in pharmaceutical industry. RD spending in the pharmaceutical industry covers a variety of activities including the following. 2 RESEARCH AND DEVELOPMENT IN THE PHARMACEUTICAL INDUSTRY in any pharmaceutical product a new molecular entity or NME or instead is an incremental modification of an existing drug. Pharmaceutical research and development consist of the investigative activities that a person or business chooses to do with the desired result of a discovery that will either create an entirely new product product line or service.
It means Research and Development. Adjusted for inflation that amount is about 10 times what the industry spent per year in the 1980s. Invention or research and discovery of new drugs.
Between 2010 and 2019 the number of new drugs approved for sale increased by 60 percent compared with the previous decade with a peak of 59 new drugs approved in 2018. The goal of this type of research is to develop newer and more effective treatments for a number of different ailments. RD can be defined as any project to resolve scientific or technological uncertainty aimed at achieving an advance in science or technology.
Development or clinical testing preparation and submission of applications for FDA approval and design of production processes for new drugs. Set the pace for innovation Faster from the lab to trials to market. With a set of aligned solutions you.
Enter your keywords. Research and Development Pharmaceutical research. It covers the management of scientific personnel management within a variety of R D organizational structures creating a climate of innovation the management of projects including the time management and communication aspects of the job.
The process starts after an initial candidate drug is identified and encompasses the rigorous research tests that determine its therapeutic suitability. Those who are working in RD-Pharmacy are termed as scientists with greater responsibilities and sharp knowledge. One of the most important sectors of the pharmaceutical industry is research and development RD which amounted to a worldwide spending of 141 billion in 2006 increased by 40 in.
Data from pharmaceutical market research firm ISR Reports reveal that research and development RD spending by 41 global pharmaceutical companies ie drug owners and primary drug developers was more than 32 billion in the first quarter of 2018 roughly a sixth of the overall 190 billion in revenues generated by those companies during the same period. In addition private laboratories work towards pharmaceutical research and development on a regular basis. The research and development RD process is a critical stage in drug development in the pharmaceutical Pharma industry.
Nearly every large pharmaceutical company has a pharmaceutical research and development department.
