About Rilzabrutinib Rilzabrutinib is an oral reversible covalent Brutons tyrosine kinase BTK inhibitor being investigated for the treatment of immune mediated diseases. PARIS April 17 2020 Sanofi will host a scientific session to present detailed data from their Phase 2b trial evaluating its investigational BTK Brutons tyrosine kinase inhibitor SAR442168 an oral brain-penetrant selective small molecule.
 Multiple Sclerosis Update Biogen Sanofi Active Adamas And Novartis Pharmalive
Multiple Sclerosis Update Biogen Sanofi Active Adamas And Novartis Pharmalive
Sanofis global head of RD John Reed Sanofi A phase 2b clinical trial of Sanofis BTK inhibitor SAR442168 in relapsing multiple sclerosis has met.
Btk inhibitor sanofi. PARIS April 23 2020 Sanofis investigational BTK Brutons tyrosine kinase inhibitor an oral brain-penetrant selective small molecule achieved both the primary and secondary endpoints in a Phase 2b trial evaluating efficacy and safety. Sanofi SA announced Thursday morning it has inked a deal with Principia Biopharma Inc. SAR442168 formerly known as PRN2246 works by inhibiting Brutons tyrosine kinase BTK a protein that is important for the activity of inflammatory cells like B.
Sanofi has announced that SAR442168 will be evaluated in four Phase 3 clinical trials in participants with relapsing and progressive forms of multiple sclerosis. Tolebrutinib formerly known as PRN2246 is an oral and selective small molecule inhibitor of the enzyme Bruton tyrosine kinase BTK that is able to cross the blood-brain barrier the. Sanofi hosted on April 23 2020 a live webcast and conference call on the detailed Phase 2b results of the investigational BTK inhibitor 168 in relapsing multiple sclerosis.
Sanofi brain-penetrant BTK inhibitor meets primary endpoint of Phase 2 trial in relapsing multiple sclerosis Sanofis BTK inhibitor will potentially be first disease-modifying therapy to address sources of multiple sclerosis MS damage in the brain Sanofi to initiate four Phase 3 clinical trials in relapsing and progressive forms of MS. To develop the South San Francisco-based biotechs oral multiple sclerosis drug PRN2246. PRN2246SAR442168 is a covalent BTK inhibitor which crosses the blood-brain barrier and is licensed to Sanofi.
Sanofi will pay 37 billion to acquire Principia Biopharma a San Francisco-based biotech firm developing small molecules that inhibit Bruton tyrosine kinase BTK. The enzyme plays an important role in B-cell maturation and function. The French pharma will pay 40 million upfront and has agreed to 756 million in milestones as well as royalties.
81 rows BTK inhibitor. In a statement Sanofi said that the acquisition will give it full control of the brain-penetrant BTK inhibitor SAR442168 making marketing more efficient and eliminating any royalty payments due. SAR442168 which Sanofi obtained in a 765 million deal with Bay Area-based Principia Biopharma is an oral brain-penetrant selective small-molecule inhibitor of BTK.
The phase 2 linked the BTK inhibitor to relative. Listing a study does not mean it has been evaluated by the US. The price represents about a 75 premium over Principias stock market value in early July before reports surfaced that Sanofi was interested in buying the firm.
BTK is involved in. Sanofi has posted the data that led it to start four phase 3 trials of its Principia Biopharma-partnered multiple sclerosis drug SAR442168. As part of their effort to identify new approaches to treating this disease Sanofi scientists are exploring ways to inhibit the activity of an enzyme known as Brutons tyrosine kinase BTK.
Primary Progressive Multiple Sclerosis PPMS Study of Brutons Tyrosine Kinase BTK Inhibitor Tolebrutinib SAR442168 PERSEUS The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The drug is designed to access the brain and spinal cord by crossing the blood-brain. Sanofis investigational BTK Brutons tyrosine kinase inhibitor an oral brain-penetrant selective small-molecule achieved both the primary and secondary endpoints in a Phase 2b trial evaluating efficacy and safety in participants with relapsing forms of multiple sclerosis.
Https Www Sanofi Com Media Project One Sanofi Web Websites Global Sanofi Com Home Media Room Press Releases 2020 2020 11 18 07 15 00 2128828 En Pdf La En
 Sanofi On Twitter The Fda Has Granted Fast Track Designation For An Investigational Btk Inhibitor For People With A Rare Blood Disorder Immune Thrombocytopenia Itp Https T Co 0trenqc0vc Https T Co Zhffiecghr
Sanofi On Twitter The Fda Has Granted Fast Track Designation For An Investigational Btk Inhibitor For People With A Rare Blood Disorder Immune Thrombocytopenia Itp Https T Co 0trenqc0vc Https T Co Zhffiecghr
 6 Sanofi Principia Biopharma Fiercepharma
6 Sanofi Principia Biopharma Fiercepharma
 Sanofi To Acquire Btk Inhibitor Firm Principia For 3 7 Billion
Sanofi To Acquire Btk Inhibitor Firm Principia For 3 7 Billion
 Sanofi Brain Penetrant Btk Inhibitor Shows Reduced Activity In Phase 2 Relapsing Ms Trial
Sanofi Brain Penetrant Btk Inhibitor Shows Reduced Activity In Phase 2 Relapsing Ms Trial
 Ir Conference Call On Phase 2b Btki 168 Trial Results Sanofi
Ir Conference Call On Phase 2b Btki 168 Trial Results Sanofi
Sanofi To Acquire Btk Inhibitor Firm Principia For 3 7 Billion
 Sanofi To Develop Principia S Btk Inhibitor For Multiple Sclerosis Global Pharma Update
Sanofi To Develop Principia S Btk Inhibitor For Multiple Sclerosis Global Pharma Update
 Sanofi Starts Off Pipeline In A Product Rilzabrutinib In Rare Skin Disease But Eyes Bigger Prize Fiercebiotech
Sanofi Starts Off Pipeline In A Product Rilzabrutinib In Rare Skin Disease But Eyes Bigger Prize Fiercebiotech
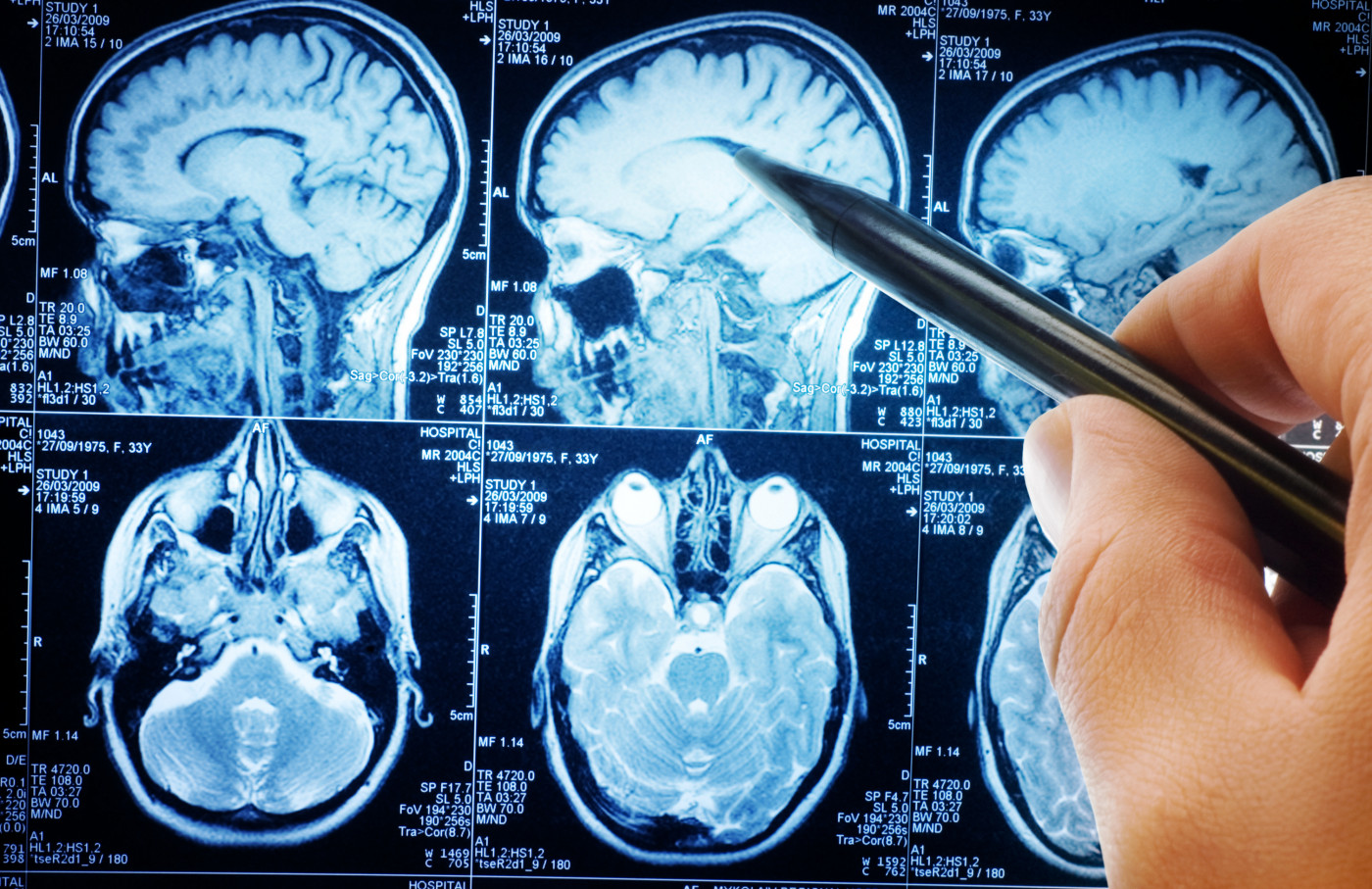 Btk Blocker Seen To Effectively Reduce Ms Brain Lesions In Phase 2 Trial
Btk Blocker Seen To Effectively Reduce Ms Brain Lesions In Phase 2 Trial
Https Www Sanofi Com Media Project One Sanofi Web Websites Global Sanofi Com Home En Investors Docs 2020 04 23 Btki Pr En Pdf La En Hash Da1556af6a05f4dd7ff48b2818c06ab2
Https Www Sanofi Com Media Project One Sanofi Web Websites Global Sanofi Com Home Media Room Press Releases 2020 2020 02 06 07 00 00 1980729 En Pdf La En
 Crossing Barriers To Deliver New Medicines Sanofi
Crossing Barriers To Deliver New Medicines Sanofi
 German Merck Boosts Autoimmune Btk Inhibitor Chase Evaluate
German Merck Boosts Autoimmune Btk Inhibitor Chase Evaluate
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.