0043mL per day 1 syringe per 14 days. We are excited to announce that Coherus has received FDA approval for Udenyca.
The approval of Udenyca is based on a review of analytical non-clinical pharmacokinetic pharmacodynamic and immunogenicity studies confirming that Udenyca is highly similar to Neulasta.

Udenyca vs neulasta. Other pegfilgrastim brands include. As an interchangeable product. Fulphila Nyvepria Udenyca Ziextenzo.
The US Food and Drug Administration FDA has approved Udencya as a biosimilar of Neulasta which means it is highly similar to and has no clinically meaningful differences from. White blood cells are vitally important in the bodys fight against infection. Nyvepria Udenyca Ziextenzo or other pegfilgrastim biosimilar product than experienced with Neulasta.
UDENYCA WAC of 4175 per prefilled syringe vs Neulasta WAC of 6231 per prefilled syringe. The list price of Neulasta has nearly tripled since approval in 2002 and now represents a 4 billion annual cost burden in the US Denny Lanfear Chairman CEO and President of Coherus BioSciences said in a press release. And the European market.
Udenyca is approved as a biosimilar to Neulasta not. The biosimilar referencing Neulasta has been approved to decrease the incidence of infection as manifested by febrile neutropenia in patients receiving myelosuppressive chemotherapy associated with a clinically significant incidence of febrile neutropenia. Neulasta Remove Neulasta from your drug comparison.
0043mL per day 1 syringe per 14 days Fulphila. 1-3 Approval based on extensive data analysis and stringent FDA requirements 4. Both of the following.
UDENYCA delivers high-quality treatment and the reliable outcomes you expect with Neulasta but with 33 cost savings per dose. 0043mL per day 1 syringe per 14 days NeulastaNeulasta Onpro. 12 J2505 Units MedAccess QL.
Fulphila pegfilgrastim-jmdb Udenyca pegfilgrastim-cbqv Nyvepria pegfilgrastim-apgf Ziextenzo pegfilgrastim-bmez Biosimilar names differ with extra letters added at the end of pegfilgrastim to help identify these products as biosimilars to the original. O History of intolerance contraindication or adverse event to Neulasta. Udenyca is a biosimilar of the reference product Neulasta.
Half Life The half-life of a drug is the time taken for the plasma concentration of a drug to reduce to half its original value. Comparing Neulasta vs Neupogen View side-by-side comparisons of medication uses ratings cost side effects and interactions. And o Physician attests that in their clinical opinion the same intolerance contraindication or adverse event would not be.
A list of biosimilars to Neulasta pegfilgrastim are. UDENYCA is an FDA-approved pegfilgrastim biosimilar administered the day after chemotherapy to lower your risk of infection. Udenyca pegfilgrastim-cbqv and Neulasta pegfilgrastim are both biological drugs containing pegfligrastim.
While dwarfed by Neulastas roughly 1 billion in quarterly sales those figures are about seven times higher than what analysts had predicted Coherus would earn from the first quarter of sales from its copycat version called Udenyca. 4-6 UDENYCA reduced patient out-of-pocket OOP costs by nearly 52 per dose 7 Contact a Coherus representative for more information about UDENYCA. Pegfilgrastim-cbqv Udenyca Coherus BioSciences a biosimilar to.
Udenyca is now approved in both the US. We believe that competition is essential in controlling burdensome price increases and Udenyca will play an important role in curbing that spend. Coherus BioSciences this week announced its biosimilar of Amgens Neulasta Udenyca will be priced at 4175 per unit a 33 discount on Neulastas list price of 6231 and below the originators average selling price of 4422.
UDENYCA provides the established outcomes you expect UDENYCA was confirmed to be highly similar to Neulasta pegfilgrastim with no clinically meaningful differences. Other filgrastim brands include. IQVIA Monthly National Sales Perspective Data.
Fulphila Neulasta Nyvepria Udenyca Ziextenzo. 144 Q5108 Units MedAccess QL. I want to thank the Coherus team our strategic partners and the FDA for this extraordinary achievement said Denny Lanfear chairman CEO and.
The FDA approved pegfilgrastim-cbqv for use by patients with cancer who are receiving myelosuppressive chemotherapy. UDENYCA stimulates the growth of neutrophils a type of white blood cell that can be depleted during chemotherapy treatment. 144 Q5111 Units MedAccess QL.
 Benefits Of Biosimilars Udenyca Pegfilgrastim Cbqv
Benefits Of Biosimilars Udenyca Pegfilgrastim Cbqv
 Benefits Of Biosimilars Udenyca Pegfilgrastim Cbqv
Benefits Of Biosimilars Udenyca Pegfilgrastim Cbqv
 Udenyca Pegfilgrastim Cbqv A Neulasta Pegfilgrastim Biosimilar
Udenyca Pegfilgrastim Cbqv A Neulasta Pegfilgrastim Biosimilar
![]() Udenyca Pegfilgrastim Cbqv A Neulasta Pegfilgrastim Biosimilar
Udenyca Pegfilgrastim Cbqv A Neulasta Pegfilgrastim Biosimilar
 Udenyca Pegfilgrastim Cbqv Injection Uses Dosage Side Effects Interactions Warning
Udenyca Pegfilgrastim Cbqv Injection Uses Dosage Side Effects Interactions Warning
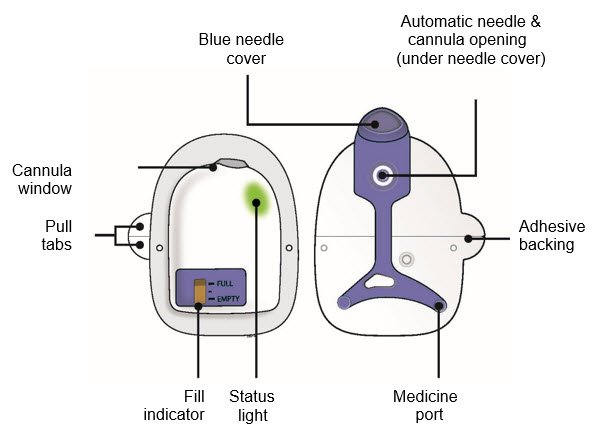 Neulasta Fda Prescribing Information Side Effects And Uses
Neulasta Fda Prescribing Information Side Effects And Uses
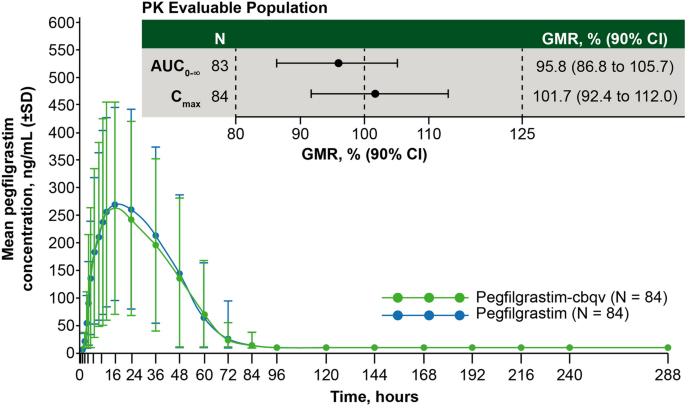


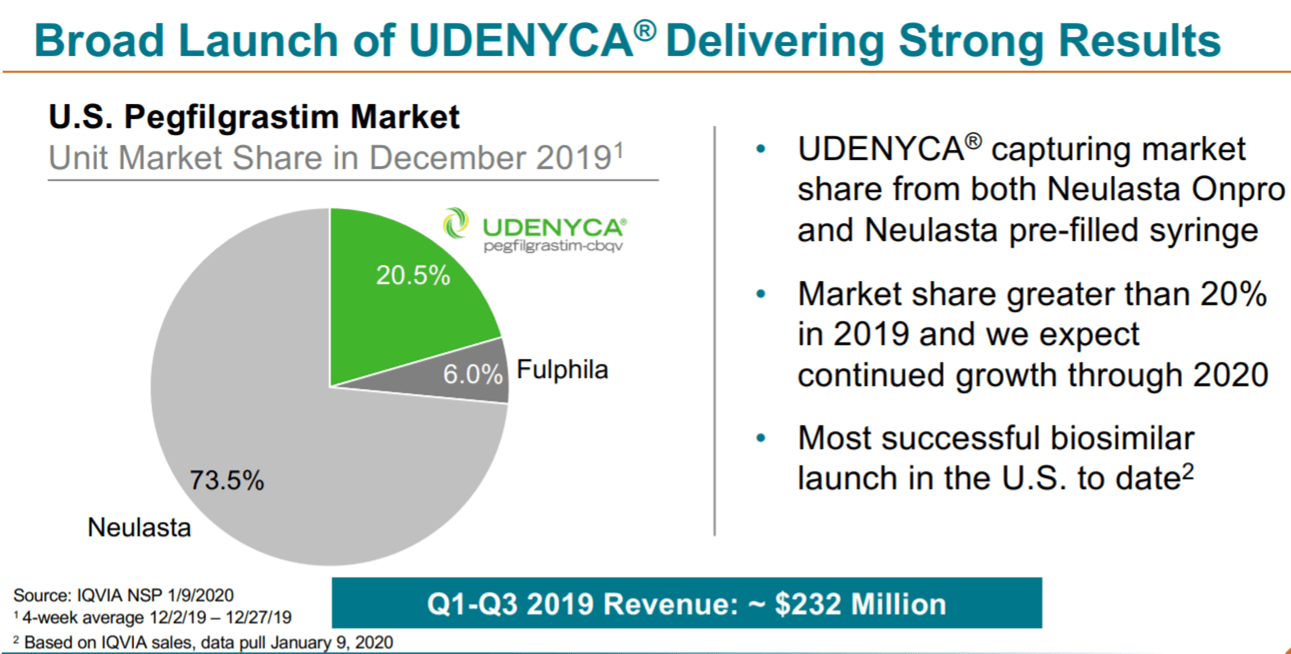


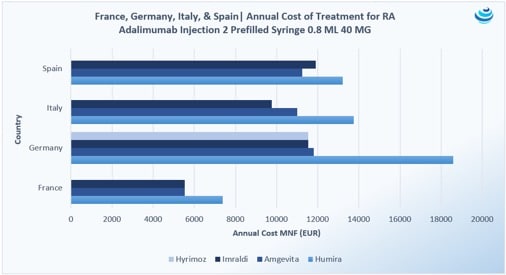

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.